A dodecanuclear copper(ii) cage self-assembled from six dicopper building units†
Abstract
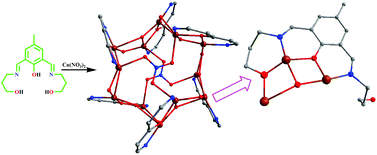
Reaction of the dinucleating phenol-based ligand, H3bpmp (2,6-bis-[(3-hydroxy-propylimino)-methyl]-4-methyl-phenol), with Cu2+ ions in the presence of a hybrid base (NEt3 and NaN3) necessary for the in situ generation of required numbers of hydroxido ions, results in the formation of a novel NO3− capped and HO− supported {Cu12} coordination complex {Cu6(μ3-OH)3(μ3-Hbpmp)3(μ3-NO3)}2(NO3)2(OH)2·2H2O·2MeOH (1). When the components are combined in right proportions (metal : ligand : NEt3 : NaN3 = 2 : 1 : 3 : 2) in MeOH, twelve Cu2+ ions assemble in a cuboctahedral geometry, containing six square and eight triangular faces around a considerable void space. Six of the eight [Cu3] triangular faces are bound by the six Hbpmp2− ligands with six free pendant propanol arms around the central hexagonal plane. X-ray structure determination indicates new geometrical features for the core formation and reveals the face-capping potential of the H3bpmp ligand for the growth of a cuboctahedral coordination cage with the support of anions like HO− and NO3−. The experimentally observed (J/kB = −173 K) strong antiferromagnetic coupling within the Cu12 complex has been justified by the DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...