Iron(iii) complexes of tripodal tetradentate 4N ligands as functional models for catechol dioxygenases: the electronic vs. steric effect on extradiol cleavage†
Abstract
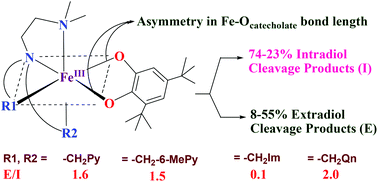
A few mononuclear iron(III) complexes of the type [Fe(L)Cl2]Cl 1–6, where L is a tetradentate tripodal 4N ligand such as N,N-dimethyl-N′,N′-bis(pyrid-2-ylmethyl)ethane-1,2-diamine (L1), N,N-diethyl-N′,N′-bis(pyrid-2-ylmethyl)ethane-1,2-diamine (L2), N,N-dimethyl-N′,N′-bis-(6-methylpyrid-2-ylmethyl)ethane-1,2-diamine (L3), N,N-dimethyl-N′-(pyrid-2-ylmethyl)-N′-(1-methyl-1H-imidazol-2-ylmethyl)ethane-1,2-diamine (L4), N,N-dimethyl-N′,N′-bis(1-methyl-1H-imidazol-2-ylmethyl)ethane-1,2-diamine (L5) and N,N-dimethyl-N′,N′-bis(quinolin-2-ylmethyl)ethane-1,2-diamine (L6), have been isolated and characterized by CHN analysis, UV-Visible spectroscopy and electrochemical methods. The complex cation [Fe(HL1)Cl3]+1a possesses a distorted octahedral geometry in which iron is coordinated by the monoprotonated 4N ligand in a tridentate fashion and the remaining three sites of the octahedron are occupied by chloride ions. The DFT optimized octahedral geometries of 1, 5 and 6 contain iron(III) with a high-spin (S = 5/2) ground state. The catecholate adducts [Fe(L)(DBC)]+, where H2DBC is 3,5-di-tert-butylcatechol, of all the complexes have been generated in situ in acetonitrile solution and their spectral and redox properties and dioxygenase activities have been studied. The DFT optimized geometries of the catecholate adducts [Fe(L1)(DBC)]+, [Fe(L5)(DBC)]+ and [Fe(L6)(DBC)]+ have also been generated to illustrate the ability of the complexes to cleave H2DBC in the presence of molecular oxygen to afford varying amounts of intra- (I) and extradiol (E) cleavage products. The extradiol to intradiol product selectivity (E/I, 0.1–2.0) depends upon the asymmetry in bidentate coordination of catecholate, as determined by the stereoelectronic properties of the ligand donor functionalities. While the higher E/I value obtained for [Fe(L6)(DBC)]+ is on account of the steric hindrance of the quinolyl moiety to coordination the lower value observed for [Fe(L4)(DBC)]+ and [Fe(L6)(DBC)]+ is on account of the electron-releasing effect of the N-methylimidazolyl moiety. Based on the data obtained it is proposed that the detachment of the –NMe2 group from the coordination sphere in the semiquinone intermediate is followed for dioxygen binding and activation to yield the extradiol cleavage product.

 Please wait while we load your content...
Please wait while we load your content...