On the origin of the spontaneous formation of nanocavities in hexagonal bronzes (W,V)O3†
Abstract
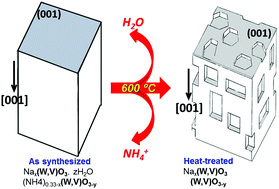
Hexagonal (W,V)O3−x oxides of high thermal stability have been synthesized hydrothermally through the intermediate products Nax(W,V)O3·zH2O and (NH4)0.33−x(W,V)O3−y. The obtained crystals show nanostructured surface via the formation of a dense population of polyhedral nanocavities self-distributed along particular crystallographic directions. Nanocavities present a regular size that ranges from 5 to 10 nm in both length and width. The synthesis process involves a significant topotactic relationship between the as-synthesized product and the desired final product and this relationship is suggested as the origin of the observed surface nanostructure. The comparison of our results with observations in different solids has allowed us to suggest that the formation of nanocavities is an extensive spontaneous process when materials are obtained by the chemical reactions of solids leading to products with defined crystallographic orientation with respect to the original compound. The characterization provides evidence regarding the potential relevance of nanocavities in the functional properties of the resulting solids.

 Please wait while we load your content...
Please wait while we load your content...