Conversion of {Fe(NO)2}10 dinitrosyl iron to nitrato iron(iii) species by molecular oxygen†
Abstract
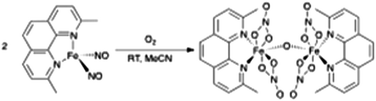
A new {Fe(NO)2}10 dinitrosyl iron complex possessing a 2,9-dimethyl-1,10-phenanthroline
- This article is part of the themed collection: New Talent: Americas

 Please wait while we load your content...
Please wait while we load your content...