The previously predicted ability of the methyl group of nitromethane to form hydrogen bonding with halides is now confirmed experimentally based on X-ray data of novel nitromethane solvates followed by theoretical ab initio calculations at the MP2 level of theory. The cationic (1,3,5-triazapentadiene)PtII complexes [Pt{HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NC5H10)N(Ph)C(NH2)
C(NC5H10)N(Ph)C(NH2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh}2](Cl)2, [1](Hal)2 (Hal = Cl, Br, I), and [Pt{HN
NPh}2](Cl)2, [1](Hal)2 (Hal = Cl, Br, I), and [Pt{HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NC4H8O)N(Ph)C(NH2)
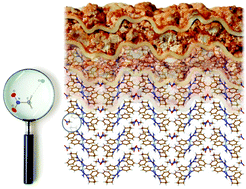
C(NC4H8O)N(Ph)C(NH2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh}2](Cl)2, [2](Cl)2, were crystallized from MeNO2-containing systems providing nitromethane solvates studied by X-ray diffraction. In the crystal structure of [1][(Hal)2(MeNO2)2] (Hal = Cl, Br, I) and [2][(Cl)2(MeNO2)2], the solvated MeNO2 molecules occupy vacant spaces between lasagna-type layers and connect to the Hal− ion through a weak hydrogen bridge via the H atom of the methyl thus forming, by means of the Hal−⋯HCH2NO2 contact, the halide–nitromethane cluster “filling”. The quantum-chemical calculations demonstrated that the short distance between the Hal− anion and the hydrogen atom of nitromethane in clusters [1][(Hal)2(MeNO2)2] and [2][(Cl)2(MeNO2)2] is not just a consequence of the packing effect but a result of the moderately strong hydrogen bonding.
NPh}2](Cl)2, [2](Cl)2, were crystallized from MeNO2-containing systems providing nitromethane solvates studied by X-ray diffraction. In the crystal structure of [1][(Hal)2(MeNO2)2] (Hal = Cl, Br, I) and [2][(Cl)2(MeNO2)2], the solvated MeNO2 molecules occupy vacant spaces between lasagna-type layers and connect to the Hal− ion through a weak hydrogen bridge via the H atom of the methyl thus forming, by means of the Hal−⋯HCH2NO2 contact, the halide–nitromethane cluster “filling”. The quantum-chemical calculations demonstrated that the short distance between the Hal− anion and the hydrogen atom of nitromethane in clusters [1][(Hal)2(MeNO2)2] and [2][(Cl)2(MeNO2)2] is not just a consequence of the packing effect but a result of the moderately strong hydrogen bonding.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NC5H10)N(Ph)C(NH2)
C(NC5H10)N(Ph)C(NH2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh}2](Cl)2, [1](Hal)2 (Hal = Cl, Br, I), and [Pt{HN
NPh}2](Cl)2, [1](Hal)2 (Hal = Cl, Br, I), and [Pt{HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NC4H8O)N(Ph)C(NH2)
C(NC4H8O)N(Ph)C(NH2)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh}2](Cl)2, [2](Cl)2, were crystallized from MeNO2-containing systems providing
NPh}2](Cl)2, [2](Cl)2, were crystallized from MeNO2-containing systems providing 

 Please wait while we load your content...
Please wait while we load your content...