Ether-based solvents significantly improved electrochemical performance for Na-ion batteries with amorphous GeOx anodes†
Abstract
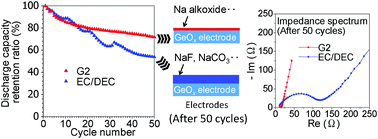
We have previously synthesized highly amorphous GeOx powder by a soft chemical method and demonstrated its performance in electrochemical Na insertion–extraction reactions. However, electrolyte decomposition caused large irreversible capacity decay in the first charge–discharge, leading to poor cycling ability. Electrolyte decomposition was accelerated by the large volume change of amorphous GeOx during charge–discharge. Herein, we report the use of ether-based solvents in Na-ion batteries with amorphous GeOx anodes. XPS measurements were used to evaluate the surface state of the electrode after cycling. The surface layer of the electrolyte decomposition products was thin and mainly composed of Na alkoxide. Thus, the electrochemical performance of this system was greatly improved owing to the significant suppression of electrolyte decomposition. Consequently, electrolytes using ether-based solvents are strong candidates for practical use in Na-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...