Polar solvent fluctuations drive proton transfer in hydrogen bonded complexes of carboxylic acid with pyridines: NMR, IR and ab initio MD study†
Abstract
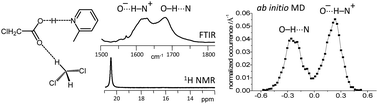
We study a series of intermolecular hydrogen-bonded 1 : 1 complexes formed by chloroacetic acid with 19 substituted pyridines and one aliphatic amine dissolved in CD2Cl2 at low temperature by 1H and 13C NMR and FTIR spectroscopy. The hydrogen bond geometries in these complexes vary from molecular (O–H⋯N) to zwitterionic (O−⋯H–N+) ones, while NMR spectra show the formation of short strong hydrogen bonds in intermediate cases. Analysis of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and asymmetric CO2− stretching bands in FTIR spectra reveal the presence of proton tautomerism. On the basis of these data, we construct the overall proton transfer pathway. In addition to that, we also study by use of ab initio molecular dynamics the complex formed by chloroacetic acid with 2-methylpyridine, surrounded by 71 CD2Cl2 molecules, revealing a dual-maximum distribution of hydrogen bond geometries in solution. The analysis of the calculated trajectory shows that the proton jumps between molecular and zwitterionic forms are indeed driven by dipole–dipole solvent–solute interactions, but the primary cause of the jumps is the formation/breaking of weak CH⋯O bonds from solvent molecules to oxygen atoms of the carboxylate group.
O stretching and asymmetric CO2− stretching bands in FTIR spectra reveal the presence of proton tautomerism. On the basis of these data, we construct the overall proton transfer pathway. In addition to that, we also study by use of ab initio molecular dynamics the complex formed by chloroacetic acid with 2-methylpyridine, surrounded by 71 CD2Cl2 molecules, revealing a dual-maximum distribution of hydrogen bond geometries in solution. The analysis of the calculated trajectory shows that the proton jumps between molecular and zwitterionic forms are indeed driven by dipole–dipole solvent–solute interactions, but the primary cause of the jumps is the formation/breaking of weak CH⋯O bonds from solvent molecules to oxygen atoms of the carboxylate group.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...