New mechanistic insight into the oxygen reduction reaction on Ruddlesden–Popper cathodes for intermediate-temperature solid oxide fuel cells
Abstract
Ruddlesden–Popper (R–P) phase materials have been investigated widely as cathode candidates for IT-SOFCs. However, widespread application of R–P phase cathodes demands further improvement in electrode activity whose progress is hindered by the limited information in the oxygen reduction reaction (ORR). The ORR mechanism for the R–P phase is therefore investigated in this paper using (LaSr)2NiO4±δ as an example. Accurate characterization of the surface oxygen exchange process is realized by developing thin and dense polycrystalline LSNO layers via a versatile spray-modified pressing method we invented before to avoid perceptible bulk diffusion contribution, surface enrichment and geometry complication. The governing factors of the ORR are identified as oxygen adsorption and incorporation based on the findings in reaction orders from electrochemical impedance spectroscopy (EIS), stoichiometry-related chemical capacitance and intrinsic anisotropic properties. The incorporation rate is proven to drastically depend on the amount of interstitial oxygen 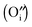 . Since the unfilled interstitial sites
. Since the unfilled interstitial sites 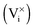 in the R–P phase serve to accommodate the adsorbed oxygen during incorporation, like vacancies in the perovskite structure
in the R–P phase serve to accommodate the adsorbed oxygen during incorporation, like vacancies in the perovskite structure 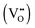 , more
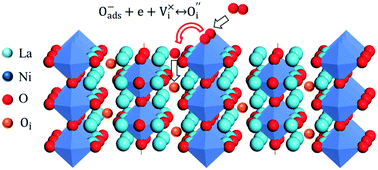
, more 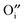 would seem to suppress the kinetics of this process. In regards to this, for the first time, a physical model is proposed to reconcile the discrepancy between the experimental results and intuitive reasoning. Based on supporting evidence, this model illustrates a possibility of how
would seem to suppress the kinetics of this process. In regards to this, for the first time, a physical model is proposed to reconcile the discrepancy between the experimental results and intuitive reasoning. Based on supporting evidence, this model illustrates a possibility of how 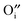 works to regulate the exchange rate, and how the contradiction between
works to regulate the exchange rate, and how the contradiction between 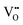 and
and 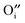 is harmonized so that the latter in the R–P structure also positively promotes the incorporation rate in the ORR.
is harmonized so that the latter in the R–P structure also positively promotes the incorporation rate in the ORR.

 Please wait while we load your content...
Please wait while we load your content...