Self-pairing of 1-methylthymine mediated by two and three Ag(i) ions: a gas phase study using infrared dissociation spectroscopy and density functional theory†
Abstract
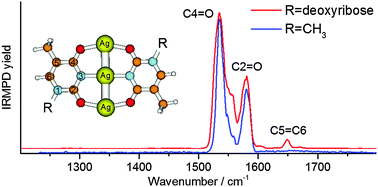
Metal base pairs of AgI cations and 1-methylthymine (1MT) or deprotonated 1-methylthymine (1MT-H) are produced and analyzed by electrospray ionization mass spectrometry (ESI-MS). Mass-selected ions of type [Ag2(1MT)(1MT-H)]+ and [Ag3(1MT-H)2]+ are interrogated by infrared multiple-photon dissociation (IRMPD) in an ion trap in the range of 1200–3700 cm−1. Supporting spectroscopic data were obtained from the investigation of the analogous 2′-deoxy-thymidine complexes which exhibit advantageously high fragment yields. By comparison with calculated linear IR spectra (obtained by density functional theory, DFT) we assign the structures and the possible isomeric forms of these metal base pairs and their dependence on the number of mediating AgI ions. Based on the observed Ag+/1MT complexes and related polarizable continuum model DFT calculations we describe the probable formation pathways in aqueous solution. The present findings pave the way for subsequent UV investigations of the multi-metal mediated base pairs.

 Please wait while we load your content...
Please wait while we load your content...