Augmented Z scheme blueprint for efficient solar water splitting system using quaternary chalcogenide absorber material
Abstract
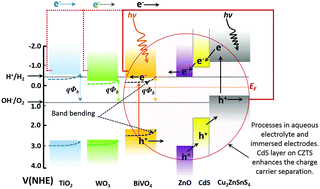
Photoelectrochemical hydrogen (H2) production from water is a key method of addressing energy needs using an environmentally friendly approach. In the last two decades we have witnessed the evolution of many different expensive catalysts, photoelectrodes and related technologies, especially those involving precious metals and use of acidic or basic electrolytes for hydrogen production. Cu2ZnSnS4 (CZTS) is a relatively new candidate in the category of efficient photocathodes, due to its high absorption coefficient and near optimal energy band gap. In this paper, we demonstrate photoelectrochemical viability of CZTS in combination with other photoanodes such as TiO2, BiVO4, and WO3 for H2 production with the use of an electrolyte of near neutral pH, a single redox mediator, and insignificant potential biasing. A systematic study was performed to understand CZTS performance with each photoanode, band energetics of CZTS with other photoanodes, impedance behavior of each photoelectrode, and utility of a CZTS photocell in place of a CZTS photocathode. Our assessment indicates that a protected CZTS photocell performs well when used in a Z-scheme containing TiO2 nanotubular array-CZTS or nanocrystalline WO3–CZTS. Preliminary experiments indicated that apart from band energetics, porosity and effective surface area of the photoanodes play a crucial role in determining the photoelectrochemical performance of the system.

 Please wait while we load your content...
Please wait while we load your content...