Protonation induced shifting of electron-accepting centers in intramolecular charge transfer chromophores: a theoretical study†
Abstract
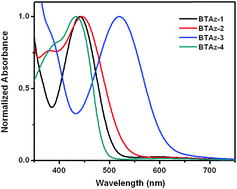
A new series of chameleonic molecules containing azulene and benzothiadiazole (BT) were designed and synthesized. In the neutral state, BT functions as an electron accepting center, while upon protonation, the electron accepting center shifts to azulene moieties, leading to a remarkable extension of absorption to the NIR region, i.e. up to 2.5 μm. The interchange between donor and acceptor characters upon protonation was confirmed by UV-vis-NIR spectral studies and supported by DFT calculations. Furthermore, the HOMO–LUMO level of ICT chromophores could be finely tailored by a different arrangement of azulenes and BTs in the molecules. The interchange between donor and acceptor characters upon protonation provides an alternative yet effective approach to fine tune optical and electronic properties of NIR chromophores.

 Please wait while we load your content...
Please wait while we load your content...