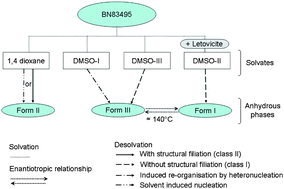
BN83495 is an API in development, used in the treatment of hormone dependent cancers. This molecule exhibits an interesting polymorphic landscape consisting of three polymorphic forms (I, II, and III), three 〈1 : 1〉 DMSO solvate polymorphs, and one 1,4-dioxane hemi-solvate. Among the three DMSO solvates, one can only be obtained in the presence of an inorganic impurity: the letovicite. Between the two polymorphic forms I and III, an enantiotropic transition has been highlighted. This solid–solid transition (Form I → Form III) seems to be controlled by the mosaicity of the particles, where both polymorphic form domains cohabit and this could remain unchanged for months. The reverse transition has not been detected in the solid state; slurrying in a solvent appears necessary to return to Form I. Each of the three polymorphs can be obtained by soaking in cold water the appropriate solvated compound. The crystal structure of each phase was determined from single crystal X-ray diffraction, except for Form II (the monotropic form under normal pressure). The analyses of the starting and final crystal structures involved in a desolvation process led to the proposal of mechanisms with and without transmission of structural information from the mother phase (i.e. the initial solvate) to the daughter phase. The presence of direct hydrogen bonds between BN83495 molecules in DMSO-I and 1,4-dioxane hemi-solvate seems to ensure the structural filiations during the desolvation mechanisms leading respectively to Form III and Form II.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...