Electrostatic properties of nine fluoroquinoloneantibiotics derived directly from their crystal structure refinements†
Abstract
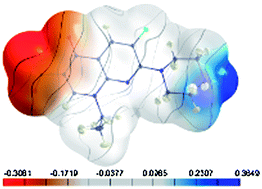
This article discusses the relevance of the similarity of the molecular electrostatic potential for rational
- This article is part of the themed collection: Crystal engineering and crystallography in the pharmaceutical industry

 Please wait while we load your content...
Please wait while we load your content...