Metal-free stereoselective annulation of quinolines with trifluoroacetylacetylenes and water: an access to fluorinated oxazinoquinolines†
Abstract
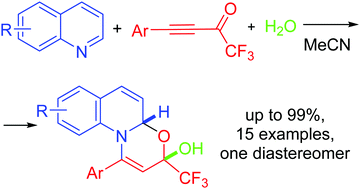
Metal-free reaction between quinolines, aryltrifluoroacetylacetylenes and water at −18 °C–rt in MeCN resulted in stereoselective assembly of trifluoromethylated oxazinoquinolines with up to 99% yield that was essentially in contrast to a similar reaction with pyridines. The annulation proceeded via the 1,3-dipolar adducts of quinolines with trifluoroacetylacetylenes followed by intramolecular cyclization involving the trifluoroacetyl group and a molecule of water.


 Please wait while we load your content...
Please wait while we load your content...