Intermolecular chemo- and regioselective aromatic C–H amination of alkoxyarenes promoted by rhodium nitrenoids†
Abstract
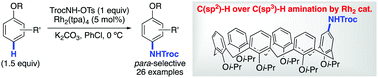
Intermolecular aromatic C(sp2)–H amination promoted by neutral rhodium nitrenoids has been developed. The reactions proceeded with various oxygen-substituted arenes (1.5 equiv.) in a chemo- and regioselective manner. The aromatic C(sp2)–H amination took place at the para position of the oxygen substituent in the presence of benzylic C(sp3)–H bonds and/or C(sp3)–H bonds α to ethereal oxygen.


 Please wait while we load your content...
Please wait while we load your content...