A NiCo2O4@Ni–Co–Ci core–shell nanowire array as an efficient electrocatalyst for water oxidation at near-neutral pH†
Abstract
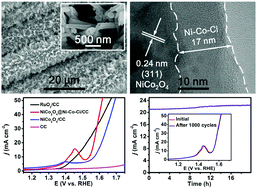
It is attractive but still remains challenging to develop efficient water oxidation electrocatalysts working in a carbonate (Ci) electrolyte. In this communication, we report that a Ni–Co–Ci layer can be developed on a NiCo2O4 nanowire array supported on carbon cloth (NiCo2O4/CC) via electrochemical surface derivation of NiCo2O4. The resulting NiCo2O4@Ni–Co–Ci core–shell nanowire array on carbon cloth (NiCo2O4@Ni–Co–Ci/CC) exhibits high activity toward water oxidation in 1.0 M KHCO3 (K–Ci, pH = 8.3) with the overpotential requirement of 309 mV to drive 10 mA cm−2. NiCo2O4@Ni–Co–Ci/CC also shows long-term electrochemical stability for 20 h and a high turnover frequency of 0.464 mol O2 s−1 at an overpotential of 600 mV.


 Please wait while we load your content...
Please wait while we load your content...