Guanidinium iodide-catalyzed oxidative α-nitroalkylation of β-ketoamides†
Abstract
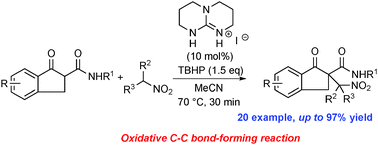
Oxidative nitroalkylation of β-ketoamides and nitroalkanes, mediated by hypoiodide generated from tert-butyl hydrogen peroxide and a catalytic amount of guanidinium iodide, afforded the corresponding α-nitroalkyl-β-ketoamides in up to 97% yield.

 Please wait while we load your content...
Please wait while we load your content...