Aerobic C–N bond activation: a simple strategy to construct pyridines and quinolines†
Abstract
Inspired by the autoxidation processes, a dioxygen induced C–N bond activation of primary alkyl amines was demonstrated toward the synthesis of pyridines and quinolines. The transition-metal free conditions with O2 as the sole oxidant make this transformation very attractive. Notably, the substrate applicability of different kinds of ketones is greatly broadened for this transformation.
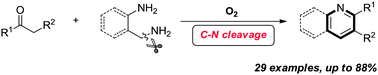


 Please wait while we load your content...
Please wait while we load your content...