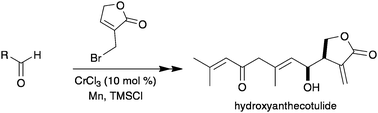
Synthesis of the anti-trypanosomal agent (±)-hydroxyanthecotulide by Cr(ii)-catalysed allylation and Meyer–Schuster rearrangement†‡
Abstract
The first synthesis of the bioactive sesquiterpene lactone hydroxyanthecotulide is achieved in 7 steps, involving a stereocontrolled Cr(II)-catalysed reaction of

 Please wait while we load your content...
Please wait while we load your content...