Alkali-metal mediated reactivity of a diaminobromoborane: mono- and bis-borylation of naphthalene versus boryl lithium or hydroborane formation†‡
Abstract
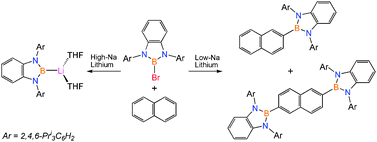
Reaction of lithium with PDABBr [PDA = C6H4-1,2-(NTripp)2, Tripp = 2,4,6-Pri3C6H2] and naphthalene afforded 2- and 2,6-borylated naphthalenes; conversely, use of high-sodium lithium (0.5% Na) afforded the lithium boryl [(PDAB)Li(THF)2]; this work establishes that main group reagents can achieve selective borylations of fused polycyclic aromatics under mild conditions in good yields.
- This article is part of the themed collection: Frontiers in Molecular Main Group Chemistry

 Please wait while we load your content...
Please wait while we load your content...