The carbonate/bicarbonate system as a pH indicator for infrared spectroscopy†
Abstract
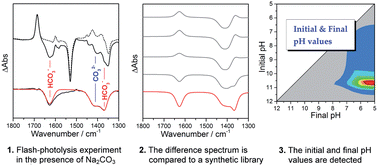
Caged compounds capable of inducing large pH-jumps upon UV illumination have represented a breakthrough in time-resolved infrared spectroscopy of acidification-triggered phenomena, but their use is hampered by the inability to control the initial pH as well as to measure the final pH in μL volumes. We have developed an experimental approach that accurately measures the initial and final pH values in pH-jump experiments. Our approach exploits the concomitant presence of two or more inorganic ions, such as carbonate and bicarbonate, that are added to the sample at a known concentration. The difference spectrum obtained in the infrared measurement is fitted to isolate the bands arising from the appearance or disappearance of either protonation state, and is then compared to a synthetic library of difference spectra generated using both qualitative (band position and width, extinction coefficient, pK) and quantitative (concentration, pathlength) parameters of the reporter ions. We have tested this approach in UV-photolysis experiments of 1-(2-nitrophenyl)ethyl sulfate in the presence of different concentrations of Na2CO3 and successfully used the infrared absorption of the carbonate and the bicarbonate ions to determine the initial and final pH values before and after the pH-jump, respectively.

 Please wait while we load your content...
Please wait while we load your content...