Controllable skeletal reorganizations in natural product synthesis
Abstract
Covering: 2016 to 2023
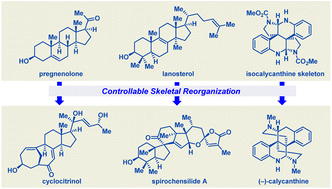
The synthetic chemistry community is always in pursuit of efficient routes to natural products. Among the many available general strategies, skeletal reorganization, which involves the formation, cleavage, and migration of C–C and C–heteroatom bonds, stands out as a particularly useful approach for the efficient assembly of molecular skeletons. In addition, it allows for late-stage modification of natural products for quick access to other family members or unnatural derivatives. This review summarizes efficient syntheses of steroid, terpenoid, and alkaloid natural products that have been achieved by means of this strategy in the past eight years. Our goal is to illustrate the strategy's potency and reveal the spectacular human ingenuity demonstrated in its use and development.


 Please wait while we load your content...
Please wait while we load your content...