Recent asymmetric synthesis of natural products bearing an α-tertiary amine moiety via temporary chirality induction strategies
Abstract
Covering: 2013 to 2023
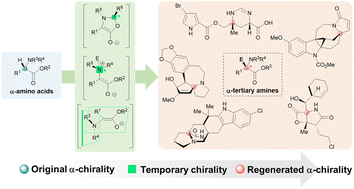
The α-tertiary amine moiety is a common structural motif in natural alkaloids and is frequently associated with intriguing biological activities and inherent synthetic challenges. A major hurdle in the total synthesis of these alkaloids is the asymmetric construction of the α-tertiary amine moiety. Temporary chirality inductions have been effective strategies employed to address this issue, particularly in natural product synthesis. The temporary chirality induction strategies in α-tertiary amine synthesis can be broadly classified into three categories based on the types of temporary chirality involved: Seebach's self-regeneration of stereocenters (SRS), C-to-N-to-C chirality transfer, and memory of chirality (MOC). This review highlights the recent advancements in temporary chirality induction strategies for the total synthesis of α-tertiary amine-containing natural products between 2013 and 2023.


 Please wait while we load your content...
Please wait while we load your content...