Breaking the activity and stability bottlenecks for acid hydrogen evolution by strong metal–support interaction between Pt nanoparticles and amorphous MoOx†
Abstract
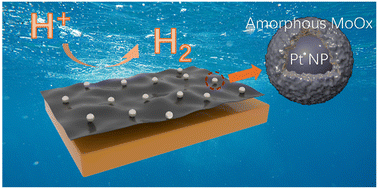
Commercial Pt/C is the best electrocatalyst for electrocatalytic hydrogen evolution due to its high activity and fast electron transfer kinetics. However, the poor stability under high current density and high cost limits its large-scale application. Here, we managed to fabricate a stable catalyst where MoOx stabilized Pt nanoparticles is supported on Ketjen Black via impregnation–annealing method. This catalyst exhibited a low overpotential of 4 mV (@10 mA cm−2) and 29 mV (@100 mA cm−2) and considerable long-term stability for 100 h toward acid hydrogen evolution reaction, outperforming commercial 20 wt% Pt/C. The excellent catalytic performances can be assigned to the existence of amorphous MoOx which can transport electrons to Pt nanoparticles and prevent Pt nanoparticles from agglomeration. This work provided a simple synthesis strategy to synthesize an amorphous MoOx protected Pt nanoparticles catalyst with good activity and stability, which can be an alternative to commercial Pt/C for acid hydrogen evolution reaction.
- This article is part of the themed collection: Emerging Investigator Series


 Please wait while we load your content...
Please wait while we load your content...