Theoretical study of Au–NX–C catalysts for H2O2 electrosynthesis via two-electron oxygen reduction reaction†
Abstract
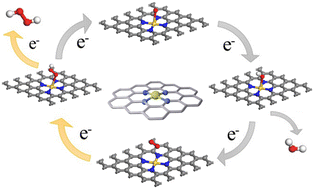
Single-atom catalysts demonstrate remarkable activity and selectivity in H2O2 electrosynthesis via two-electron oxygen reduction reaction (ORR). Currently, the predominant strategy employed to enhance the selectivity for H2O2 production and subsequently improve the overall H2O2 yield involves dispersing metal sites exhibiting high activity. However, a noteworthy research gap exists in enhancing catalytic activity and H2O2 yield by modifying low-activity dispersed metal sites. In this study, we introduce Au–N4–C as a highly promising catalyst for the electrochemical production of H2O2. Using density functional theory and first-principles analysis, we analyze the active centers, conductivity, catalytic activity, and selectivity of Au–NX–C catalysts with distinct N-vacancy structures. The findings demonstrate that the Au–N4–C structure exhibits desirable conductivity and robust Au–O bonding upon O2 adsorption, resulting in superior 2e− ORR catalytic activity and favorable 2e− ORR selectivity. These results validate the effectiveness of the proposed method in enhancing the catalytic performance of single-atom catalysts, thereby enabling more efficient production of H2O2 through adjustment of the coordination environment of the metal active center. Furthermore, these results offer valuable insights into the exploration of single-atom catalysts for the electrochemical production of H2O2.


 Please wait while we load your content...
Please wait while we load your content...