A panoramic view on synthetic applications of α-oxothioamides: a highly regioselective synthesis of 2-acyl-4-(het)arylthiazoles and thioethers†
Abstract
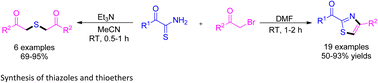
Highly regioselective synthesis of 2-acyl-4-(het)arylthiazoles and thioethers by the reaction between α-oxothioamides and α-bromoketones in the absence of base in DMF and in the presence of triethylamine in acetonitrile, respectively, has been reported. This thiazole synthesis is an important extended work of the Hantzsch thiazole synthesis, which overcomes the drawbacks of earlier reported methods. The probable mechanisms for the formation of thiazoles and thioethers are also presented.


 Please wait while we load your content...
Please wait while we load your content...