New insights into the base catalyzed depolymerization of technical lignins: a systematic comparison†
Abstract
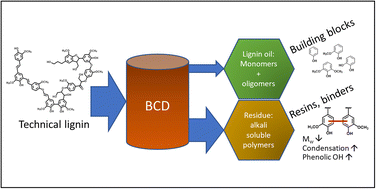
A first systematic approach on the base catalyzed depolymerization (BCD) of five technical lignins derived from various botanical origins (herbaceous, hardwood and softwood) and covering the main three industrial pulping methods (soda, kraft and organosolv) is reported. This study provides a first of its kind in-depth quantification and structural characterization of two main BCD fractions namely lignin oil and lignin residue, describing the influence of the BCD process conditions. Depolymerization is evaluated in terms of lignin conversion, lignin oil yield, phenolic monomer selectivity and the production of lignin residue and char. Lignin oils were extensively characterized by size exclusion chromatography (SEC), GC-MS, GC-FID, 13C-NMR, HSQC NMR and elemental analysis. GC × GC-FID was used to identify and quantify distinct groups of monomeric compounds (methoxy phenols, phenols, dihydroxy-benzenes) in the lignin oil. The lignin oil yields (w/w) ranged from 20–31% with total monomer contents ranging from 48 to 57% w/w. SEC analysis indicated the presence of dimers/oligomers in the lignin oil, which through HSQC NMR analysis were confirmed to contain new, non-native interunit linkages. 13C NMR analyses of the lignin oils suggest the presence of diaryl type linkages (i.e. aryl–aryl, aryl C–O) evidencing deconstruction and recombination of lignin fragments during BCD. Irrespective of the lignin source, a residue, often regarded as ‘unreacted’ residual lignin was the main product of BCD (43 to 70% w/w). Our study highlights that this residue has different structural properties and should not be considered as unreacted lignin, but rather as an alkali soluble condensed aromatic material. HSQC, DEPT-135, 13C, and 31P NMR and SEC analyses confirm that the BCD residues are indeed more condensed, with increased phenolic hydroxyl content and lower molecular weights compared to all feed lignins. Subsequent BCD of solid residual fractions produced only low oil yields (6–9% w/w) with lower phenolic monomer yields (4% w/w) compared to original lignin, confirming the significantly more recalcitrant structure. Our study improves the overall understanding of the BCD process, highlights important feedstock-dependent outcomes and ultimately contributes to the complete valorization of BCD-derived lignin streams.


 Please wait while we load your content...
Please wait while we load your content...