Metal-free direct C-6–H alkylation of purines and purine nucleosides enabled by oxidative homolysis of 4-alkyl-1,4-dihydropyridines at room temperature†
Abstract
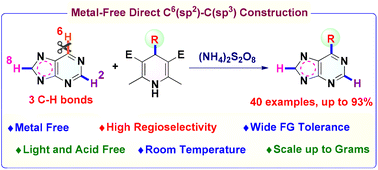
Herein we report the application of 4-alkyl-1,4-dihydropyridines (DHPs), which are easily prepared from inexpensive aldehydes in one step, for the direct site-specific C–H alkylation of purines and purine nucleosides. Despite there being three active C(sp2)–H bonds (C-2–H, C-6–H, and C-8–H) in the structure, the reactions still show high regioselectivity at the purinyl C-6–H position. Importantly, the reactions successfully avoid the use of transition metal catalysts and additional acids. Meanwhile, the protocols are not sensitive to moisture and require only persulfate as an oxidant. Besides, this method displays broad functional group compatibility and is easy to scale up. Notably, pharmaceutical purines, e.g. the natural product 6-hydroxymethyl nebularine isolated from basidiomycetes, can be smoothly prepared using this protocol.


 Please wait while we load your content...
Please wait while we load your content...