Hydrogen production via reaction of metals with supercritical water
Abstract
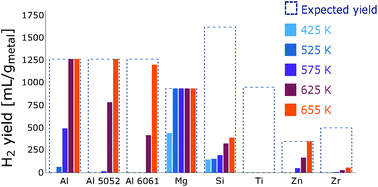
Reactive metals have been identified as promising energy dense, sustainable energy carriers suitable for long-duration energy storage. The lack of such energy storage is a bottleneck in the transition towards an energy system compatible with arresting climate change. In this work, aluminum alloy plates, magnesium slugs, zinc slugs, and silicon, titanium, and zirconium powders are reacted with high-temperature liquid water and supercritical water (655 K, 230 bar) to produce hydrogen and heat. With the exception of titanium, which showed virtually no reactivity with water, it was found that, in all cases, reaction efficiency increased with temperature. Silicon and zirconium showed poor reactivity, while full hydrogen yield was observed in the supercritical regime for the aluminum alloys, magnesium and zinc. The magnesium slugs were the most reactive of the materials tested, reacting fully with liquid water at 525 K. A link between oxide solubility and reactivity with water is proposed.


 Please wait while we load your content...
Please wait while we load your content...