The methodology for preparing domperidone: strategies, routes and reaction processes
Abstract
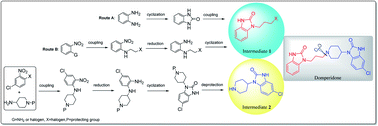
Domperidone is a powerful peripheral dopamine receptor antagonist; however, a systematic review of the synthetic methods and processes of this drug has not been reported so far. This review summarizes the synthetic strategies, synthetic routes and reaction processes of domperidone in detail. Domperidone can be synthesized from the coupling reaction of two benzimidazolone derivatives (intermediates 1 and 2). Intermediate 1 can be prepared by two synthetic routes: the cyclization of o-phenylenediamine with carbonyl reagents followed by coupling with 1,3-dihalopropane, and the coupling reaction of o-halo or o-amino substituted nitrobenzene with 1,3-disubstituted propane followed by reduction and cyclization. The latter route avoids the production of di-substituted by-products and has higher reaction selectivity. Intermediate 2 is synthesized by coupling substituted nitrobenzene with 4-aminopiperidine followed by reduction and cyclization, which is similar to the synthetic route of intermediate 1. Understanding the advantages and drawbacks of these synthetic methodologies would provide insights for the development of new strategies to prepare domperidone. Moreover, the methods used to synthesize domperidone can provide alternative approaches in the preparation of drugs or compounds with similar structure.
- This article is part of the themed collection: 2022 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...