Tuning the catalytic properties of La–Mn perovskite catalyst via variation of A- and B-sites: effect of Ce and Cu substitution on selective catalytic reduction of NO with NH3
Abstract
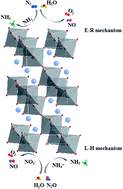
Perovskites with flexible structures and excellent redox properties have attracted considerable attention in industry, and their denitration activities can be further improved with metal substitution. In order to investigate the effect of Ce and Cu substitution on the physicochemical properties of perovskite in NH3-SCR system, a series of La1−xCexMn1−yCuyO3 (x = 0, 0.1, y = 0, 0.05, 0.1, 0.2, 0.4) catalysts were prepared by citrate sol-gel method and employed for NO removal in the simulated flue gas, and the physical and chemical properties of the catalysts were studied using XRD, SEM, BET, XPS, DRIFT characterizations. The Ce substitution on A-site cation of LaMnO3 can improve the denitration activity of the perovskite catalyst, and La0.9Ce0.1MnO3 displays NO conversion of 86.7% at 350 °C. The characterization results indicate that the high denitration activity of La0.9Ce0.1MnO3 is mainly attributed to the larger surface area, which contributes to the adsorption of NH3 and NO. Besides, the appropriate Cu substitution on B-site cation of La0.9Ce0.1MnO3 can further improve the denitration activity of perovskite catalyst, and La0.9Ce0.1Mn0.8Cu0.2O3 displays the NO conversion of 91.8% at 350 °C. Although the specific surface area of La0.9Ce0.1Mn0.8Cu0.2O3 is lower than La0.9Ce0.1MnO3, the Cu active sites and the Ce3+ contents are more developed, making many reaction units formed on the catalyst surface and redox properties of catalyst improved. In addition, strong metal interaction (Ce4+ + Mn2+ + Cu2+ ↔ Ce3+ + Mn3+/Mn4+ + Cu+) and high concentrations of chemical adsorbed oxygen and lattice oxygen both strengthen the redox reaction on catalyst surface, thus contributing to the better denitration activity of La0.9Ce0.1Mn0.8Cu0.2O3. Therefore, appropriate cerium and copper substitution will markedly improve the denitration activity of La–Mn perovskite catalyst. We also reasonably conclude a multiple reaction mechanism during NH3-SCR denitration process basing on DRIFT results, which includes the Eley–Rideal mechanism and Langmuir–Hinshelwood mechanism.


 Please wait while we load your content...
Please wait while we load your content...