Synthesis of mixed musks via Eschenmoser–Tanabe fragmentation, enyne metathesis and Diels–Alder reaction as key steps†
Abstract
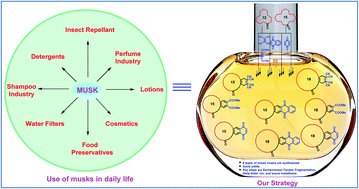
Musk analogues containing different macrocyclic ring systems as well as different annulated ring systems were synthesised by a simple and useful strategy. This strategy includes Eschenmoser–Tanabe fragmentation, enyne metathesis and Diels–Alder reaction as key steps. Starting from easily available (n) macrocyclic ketones, (n + 3) macrocyclic systems were assembled using the basic organic reactions.


 Please wait while we load your content...
Please wait while we load your content...