Isotopic labelling experiments and enzymatic preparation of iso-casbenes with casbene synthase from Ricinus communis†
Abstract
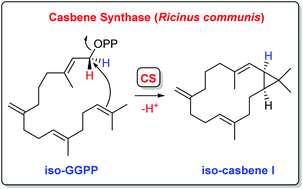
Isotopic labelling experiments gave insights into the enzyme mechanism of casbene synthase from Ricinus communis, showing a clear stereochemical course for the cyclisation reaction, in agreement with the reported absolute configuration of casbene. Two oligoprenyl diphosphate analogs with shifted double bonds were synthesised and enzymatically converted with casbene synthase to yield casbene isomers. Their absolute configurations were evident from a terpene cyclisation through the same stereochemical course as for casbene. Additional labelling experiments gave insights into the EI-MS fragmentation mechanism of casbene.


 Please wait while we load your content...
Please wait while we load your content...