Selective 1,4-arylsulfonation of 1,3-enynes via photoredox/nickel dual catalysis†
Abstract
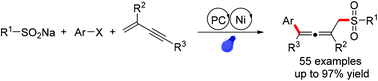
A photoredox/nickel-catalyzed selective 1,4-arylsulfonation of 1,3-enynes to access structurally diverse sulfone-containing allenes has been established. This radical cascade transformation featured easy manipulation, mild conditions, low catalyst loading, broad substrate scope, and large-scale synthesis. The preliminary mechanistic studies indicated a possible radical-relay process enabled by the radical capture of nickel(0) species.


 Please wait while we load your content...
Please wait while we load your content...