From α-keto acids to nitrile oxides enabled by copper nitrate: a facile access to fused isoxazolines†
Abstract
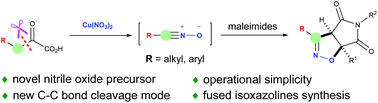
α-Keto acids were unprecedentedly employed as novel precursors of nitrile oxides on treatment with copper nitrate, which reacted with maleimides via [3 + 2] dipolar cycloaddition leading to pharmacologically interesting fused isoxazolines. This approach offers a unique strategy and complementary method for the convenient generation of alkyl substituted nitrile oxides, compared with previously reported copper nitrate-mediated alkenes or alkynes transformations. Different from the well-documented decarboxylation or decarbonylation of α-keto acids, the mechanistic studies revealed that α-keto acids went through a novel carbon–carbon bond cleavage resulting in the key gem-dinitroalkane intermediates, which were further transformed to nitrile oxides for the following 1,3-dipolar cycloadditions.


 Please wait while we load your content...
Please wait while we load your content...