Facile access to the 2,2-difluoro-2,3-dihydrofuran skeleton without extra additives: DMF-promoted difluorocarbene formation of ClCF2CO2Na†
Abstract
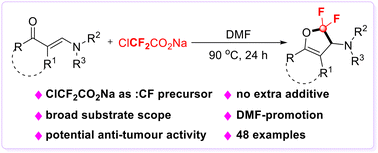
A practical and facile difluorocarbene-triggered cycloaddition reaction of enaminones was developed, which delivered 2,2-difluoro-2,3-dihydrofurans with a broad substrate scope. Notably, the reaction proceeded smoothly without any extra additives. Readily available sodium chlorodifluoroacetate (ClCF2CO2Na, SCDA) served as a difluorocarbene precursor in this transformation through DMF-promotion. Moreover, it is proved that the 2,2-difluoro-2,3-dihydrofuran derivatives exhibit potential antiproliferative activity against human tumour cells HeLa, MCF7 and HepG2.


 Please wait while we load your content...
Please wait while we load your content...