Metal-free visible-light-driven cascade cyclization reaction to synthesize 2-oxindoles via benzoyl and phenylsulfinyl radicals with acrylamide derivatives†
Abstract
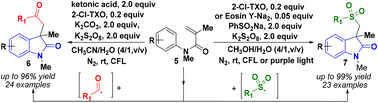
A metal-free visible-light-driven cascade cyclization reaction to synthesize 3-methyl-3-acetophenone-2-oxindoles and 3-methyl-3-(methylsulfonyl)benzene-2-oxindoles in yields up to 96% and 99%, via benzoyl and phenylsulfinyl radicals with acrylamide derivatives is reported, respectively. Extensive studies, including gram-scale, radical capture and isotope experiments, were performed to indicate that the reaction may involve a radical process.


 Please wait while we load your content...
Please wait while we load your content...