Brønsted acid-catalyzed enantioselective addition of 1,3-diones to in situ generated N-acyl ketimines†
Abstract
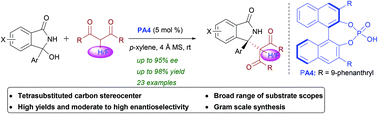
A Brønsted acid-catalyzed asymmetric Mannich-type addition of 1,3-diones to cyclic N-acyl ketimines is reported for the synthesis of enantioenriched isoindolinones. Various dicarbonyl-substituted isoindolinones bearing a quaternary carbon stereocenter were synthesized with excellent yields (up to 98%) and moderate to high enantioselectivities (up to 95% ee), and most of them possess a fluorine atom at the reactive center. Furthermore, the synthetic utility of the protocol has been demonstrated by the debenzoylation of the product.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...