Synthesis of methylene-bridged α,β-unsaturated ketones: α-Csp3–H methylenation of aromatic ketones using Selectfluor as a mild oxidant†
Abstract
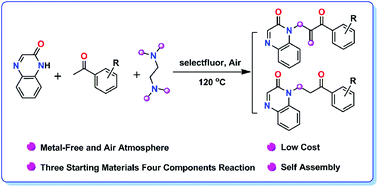
A three starting material four component reaction (3SM-4CR) is developed for the synthesis of α,β-unsaturated ketones and β-amino ketones in good yields. The reaction employs tetramethylethylenediamine (TMEDA) as a methylene and terminal olefin source, and Selectfluor as a mild oxidant. TMEDA worked as a dual synthon to provide two carbons in this metal-free transformation process. The scope and versatility of the methods have been demonstrated with 23 examples. A Selectfluor-promoted oxidative reaction mechanism is proposed based on the results of the experimental studies.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...