Iron-catalyzed ring-opening of cyclic carboxylic acids enabled by photoinduced ligand-to-metal charge transfer†
Abstract
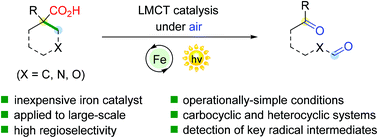
A decarboxylative ring-opening protocol of cyclic tertiary carboxylic acids via iron-catalyzed photoinduced ligand-to-metal charge transfer (LMCT) is described. This reaction enables the preparation of 1,n-dicarbonyl compounds through the homolytic C–C bond cleavage of unstrained carbocyclic and heterocyclic ring systems. This method exhibits a lot of synthetic advantages including mild conditions, simple operation, and convenience of amplification. Mechanistic studies support the generation of peroxy radical species via oxygen capture followed by radical fragmentation.


 Please wait while we load your content...
Please wait while we load your content...