A convenient transformation of alkynes to α-hydroxy-α-CF3-γ-ketoester derivatives via H3PO4-catalyzed tandem hydration and aldol condensation†
Abstract
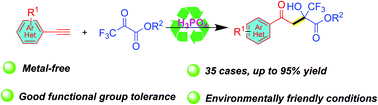
A robust metal-free catalytic strategy for the rapid construction of the valuable α-hydroxy-α-CF3-γ-ketoester skeleton with cheap and easily available stock chemicals (alkynes and trifluoropyruvate) as the starting material is described. This protocol shows very good functional group tolerance and is easily scaled up. Importantly, compared with transition metal-catalyzed methods, this strategy is made more environmentally friendly by employing a catalytic amount of H3PO4 as the catalyst.


 Please wait while we load your content...
Please wait while we load your content...