Insights from multi-spectroscopic analysis and molecular modeling to understand the structure–affinity relationship and the interaction mechanism of flavonoids with gliadin†
Abstract
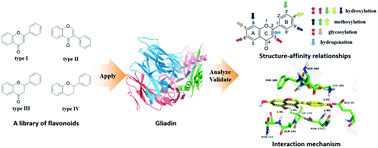
Gliadin, as a main component of wheat storage protein, is used as a drug encapsulation and delivery system owing to its specific characteristics. Flavonoids are regarded as active natural products with a variety of pharmacological effects. In this study, an integrated method including UV-vis, fluorescence, and FT-IR spectroscopy and molecular modelling was applied to explore the structure–affinity relationship and the interaction nature between a library of flavonoids and gliadin. The characteristic UV-vis spectral changes of gliadin mediated by flavonoids with absorption bands at 218 and 278 nm demonstrated the existence of an interaction depending on generating the ground-state complexes. Fluorescence quenching results showed that the intrinsic fluorescence of gliadin could be effectively quenched by flavonoids coupled with the formation of flavonoid–gliadin complexes through the static quenching mechanism. The structure–affinity relationship revealed the critical structural elements associated with the binding affinity on gliadin and underlined the favorable substituents at the specific positions of flavonoid skeletons leading to a stronger binding potency. From the analysis of synchronous fluorescence spectra, flavonoids could cause the conformation change of gliadin and impact the microenvironment around TYR and TRP residues. Moreover, the ANS fluorescent probe assay suggested that these flavonoids also influenced the surface hydrophobicity of glaidin based on the further exposure or blocking of hydrophobic domains. Molecular modelling was subsequently performed and illustrated the proposed binding conformation of flavonoids on gliadin. Combined with the FT-IR spectra, these results further confirmed the important role of hydrophobic interactions and hydrogen bonds in their binding process.


 Please wait while we load your content...
Please wait while we load your content...