One-step fabrication of MoS2/Ni3S2 with P-doping for efficient water splitting†
Abstract
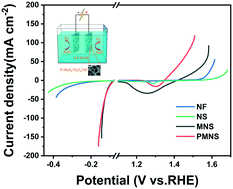
Designing and building high-performance bifunctional electrocatalysts is critical for water splitting. Herein, an efficient P-doped molybdenum disulfide/nickel subsulfide (abbreviated as P-MoS2/Ni3S2/NF hereafter) catalyst was rationally prepared through a facile one-step hydrothermal process. The heterogeneous structure formed by the combination of MoS2 and Ni3S2 can improve the electrolytic performance of water in an alkaline medium. The addition of a P source in the reaction reduces the agglomeration of the catalyst, and the electronic structure of MoS2/Ni3S2 is simultaneously optimized by the doped P atoms in MoS2, thus promoting conductivity and increasing the valence state of the Ni species. Low overpotentials of only 136 mV and 95 mV are required to generate 10 mA cm−2 for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), respectively. Most significantly, when P-MoS2/Ni3S2/NF is used as the cathode and anode for the electrolysis of water, a voltage of 1.48 V is required to produce 10 mA cm−2. This work provides feasible ideas for the rational construction of bifunctional catalysts with outstanding performance, which may offer a reference for designing non-precious electrocatalysts for water splitting.


 Please wait while we load your content...
Please wait while we load your content...