Structure directing roles of weak noncovalent interactions and charge-assisted hydrogen bonds in the self-assembly of solvated podands: an example of an anion-assisted dimeric water capsule†
Abstract
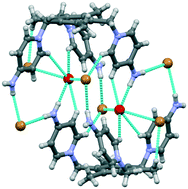
Crystal structures of two new podand molecules (1 and 2) synthesized from 1,3,5-tris(bromomethyl)mesitylene and two bromide salts of tris(4-amino-N-ethylbenzamide)amine (3) were elucidated to witness the structure directing roles of weak noncovalent interactions (C–H and π–π interactions) and/or charge-assisted hydrogen bonds in the organized self-assembly of the solvated podands. Podand 1 crystallizes from acetone as [1·(CH3)2CO], and shows self-assembly via C–H⋯O hydrogen bonds and π–π interactions to generate a well-organized three-dimensional framework. Podand 2 crystallizes from methanol–water as [2·H2O], and shows the formation of a hydrogen bonded dimeric capsular assembly where the encapsulated water molecules interact with aromatic C–H protons and amine N–H protons of the tripodal scaffold, and also with bromide counter-anions which are located outside the tripodal cavity. Unlike [2·H2O], no lattice water is encapsulated within the molecular cavity of protonated podand 3 in its dibromide salt [(3H2)2+·(Br−)2·(4H2O)] and tetrabromide salt [(3H4)4+·(Br−)4·(H2O·CH3OH)] due to intramolecular hydrogen bonding and π–π interactions between the pendent arms. The crystal structures of both dibromide and tetrabromide salts showed self-assembly primarily via charge-assisted N–H⋯O and N–H⋯Br− hydrogen bonds, as the number of N–H hydrogen bonds far exceeds the number of C–H hydrogen bonds in both cases. The 1H-NMR spectra of the dibromide and tetrabromide salts showed variable shifts of the aromatic –CH proton signals in both DMSO-d6 and D2O, indicating the different degrees of protonation in podand 3. The relative contributions of different types of interactions (in percentage) observed in the crystal structures of the podands were obtained from Hirshfeld surface analysis.


 Please wait while we load your content...
Please wait while we load your content...