An investigation on 3-acetyl-7-methoxy-coumarin Schiff bases and their Ru(ii) metallates with potent antiproliferative activity and enhanced LDH and NO release†
Abstract
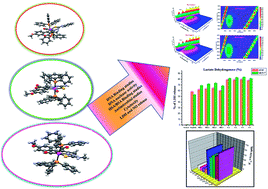
New cyclometallated ruthenium(II) complexes of 3-acetyl-7-methoxycoumarin-4N-substituted thiosemicarbazones were synthesized and characterized by analytical and spectral techniques. The crystal structures of the ligands H2L1–3 and complexes (1, 2 and 4) were confirmed by X-ray crystallography. The analysis showed that the ligands have undergone C–H activation at the C(4) carbon of the pyrone ring and acted in a tridentate fashion by binding through C, N and S atoms. CT-DNA and protein (BSA/HSA) binding studies were carried out to analyze their interaction with biomolecules. Good binding affinity with DNA was observed with intercalative binding mode, which was further confirmed by EB displacement and viscosity measurement studies. The quenching mechanism with BSA/HSA was found to be static. Three dimensional (3D) fluorescence measurements were carried out to validate the micro environmental changes in the serum albumins. Their antioxidant propensity and antimicrobial study insisted that the compounds displayed good spectrum of activity. Evaluation of their anticancer potential against MCF-7 (human breast cancer) and A549 (human lung carcinoma) cell lines revealed that the complexes exhibited better activity than the ligands and cisplatin. Further, the results of LDH and NO release assays supported the cytotoxic nature of the compounds. The non-toxic nature of the compounds was established by testing against the non-cancerous cell line HaCaT (human normal keratinocyte).


 Please wait while we load your content...
Please wait while we load your content...