Facile synthesis of macrocyclic peptide toxins of GpTx-1 and its analogue†
Abstract
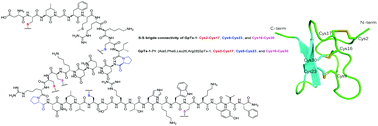
GpTx-1, a 34-residue peptide cross-linked by three disulfide bridges, originally isolated from the venom of the tarantula Grammostola porter, and its analogue GpTx-1-71 ([Ala5,Phe6,Leu26,Arg28]GpTx-1) are highly potent pharmaceutical inhibitor candidates to voltage-gated sodium channels, currently in preclinical development. GpTx-1 and its analogue for drug development had previously been obtained by adopting a tedious stepwise solid phase peptide synthesis strategy. Herein, we present a flexible and highly practical strategy by converging three segments based on C-terminal proline residues. This approach provided a reliable and convenient synthetic route which could greatly facilitate GpTx-1 based drug developments for pain relief. Solution NMR structural analysis and electrophysiology evaluation verified the compositional and functional characteristics of the two macrocyclic peptides, which are consistent with previous studies.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...