Diastereoselectivity in a cyclic secondary amine catalyzed asymmetric Mannich reaction: a model rationalization from DFT studies†
Abstract
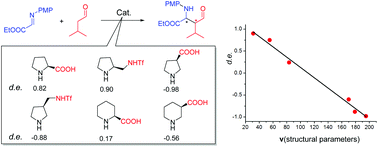
A variation in diastereoselectivity is rationalized for cyclic secondary amine catalyzed asymmetric Mannich reactions, by systematic DFT studies on a series of representative catalysis systems. Computational results well explain the experimentally observed stereoselectivities for all the systems. Structural factors that influence the stereoselectivity are elucidated based on transition state analysis. Three structural parameters including two lengths L1 and L2, and a dihedral angle D1 of intermediates are found to be able to concisely correlate the relationship between structures and diastereoselectivities. A quantitative structure-stereoselectivity relationship (QSSR) model containing these three parameters is proposed to correlate the diastereoselectivity of Mannich reactions catalyzed by cyclic secondary amines. The model rationalization study could serve as an interesting example to understand the stereoselectivities and to advance catalyst design in asymmetric organocatalysis.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...