Isolation and biosynthesis of labdanmycins: four new labdane diterpenes from endophytic Streptomyces†
Abstract
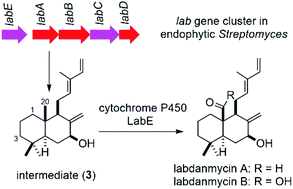
Chemical investigation of Streptomyces sp. KIB 015, an endophyte from the plant Panax notoginseng, led to the discovery of two novel labdane diterpenes, labdanmycins A (1) and B (2). In order to reveal the biosynthesis of labdanmycins, the genome of S. sp. KIB 015 was sequenced and a lab gene cluster encoding GGPP synthase, class II cyclase, class I cyclase, and cytochrome P450s was confirmed to be involved in labdanmycin biosynthesis by heterologous expression and gene inactivation experiments. Biogenetic precursor raimonol (3) was isolated from the gene labE inactivation mutant strain S. sp. KIB 015 ΔlabE, which suggested that P450 enzyme LabE is responsible for oxidizing the C-20 methyl group of 3 to an aldehyde or carbonyl group to afford 1 and 2. Additional two new labdane diterpenes (4 and 5) were also isolated from the mutant S. sp. KIB 015 ΔlabE. The chemical structures of 1–5 were elucidated on the basis of extensive NMR and MS spectroscopic analyses.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...