Formation of ozone by solid state reactions
Abstract
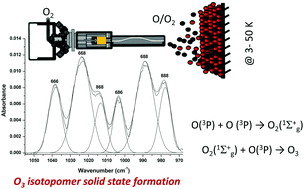
We studied the isotopic composition of ozone formed at low (3–10 K) temperature via O + O2 solid state reactions using a partially dissociated 16O/16O2 : 18O/18O2 = 1 : 1 mixture. The ozone ice has an isotopic abundance that differs from the statistical one and from gas phase studies. Ozone formation is influenced by the competition of the production of O2 (O + O or O + O3) vs. O3 (O + O2) and by the energy released in the O + O reaction. The exothermicity of the O + O reaction helps to overcome the barrier of the O + O2 reaction. Heating the ozone ice past 50 K brings about a transformation from amorphous to crystalline ice. The formation of ozone on water ice yields a blue shift of IR bands, and the yield of formed O3 increases up to the sample temperature of 100 K. When 18O/18O2 is deposited on H216O ice, formation of 18O18O16O is detected. We propose that the exothermicity of the reaction 18O + 18O drives water dissociation (16O + H2) followed by ozone formation (16O + 18O2 → 16O18O18O).
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
