Threshold for shattering fragmentation in collision-induced dissociation of the doubly protonated tripeptide TIK(H+)2
Abstract
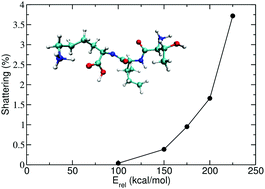
In a recent direct dynamics simulations of the collision induced dissociation (CID) of the doubly protonated tripeptide threonine–isoleucine–lysine and threonine–leucine–lysine ions, TIK(H+)2 and TLK(H+)2, a shattering fragmentation mechanism was found, in which the ion fragmented upon impact with N2 (Z. Homayoon et al., Phys. Chem. Chem. Phys., 2018, 20, 3614). In using models to interpret experiments of biological ion CID, it is important to know the collision energy threshold for the shattering mechanism. In the work presented here, direct dynamics simulations were performed to study shattering fragmentation versus the collision energy (Erel) for N2 + TIK(H+)2. From the probability of shattering fragmentation and the minimum energy transfer for fragmentation versus Erel, a threshold of ∼55 kcal mol−1 was identified for N2 + TIK(H+)2 shattering fragmentation. This threshold is substantially higher than the lowest activation energy of 14.7 kcal mol−1, found from direct dynamics simulations, for the thermal dissociation of TIK(H+)2.


 Please wait while we load your content...
Please wait while we load your content...
